
Last Updated on
August 19, 2022
By
Excedr
2′-deoxycytidine 5′-triphosphate (or dCTP) is a nucleoside triphosphate (a nucleotide), containing cytosine as its pyrimidine base. It also has deoxyribose sugar attached to three phosphate units. Its chemical formula is C9H16N3O13P3 and has a molecular weight of 467.1569 g/mol. The cas number of the compound is 2056-98-6—this number is given by the Chemical Abstracts Service (CAS) to refer directly to the compound’s information.
In the double-stranded DNA, dCTP is paired with dGTP, forming three hydrogen bonds, whereas dATP and dTTP make a pair by forming two hydrogen bonds. So, the GC content in the strand contributes maximum to the stability of the structure and determines its physical properties, such as its melting temperature.
Some other derivatives of cytosine (such as 5-methyl-cytosines that is formed after methylation and dUTP that result after bisulfite treatment), also have essential functions to perform in organisms.
Bisulfite treatments and the use of methylation-sensitive restriction enzymes (with or without methylation treatment) are two methods that are utilized to detect the methylation of dCTP in genomic DNA.
This article provides a complete overview of what dCTP is and its uses in labs and the body of other organisms. Furthermore, it covers its structural representations and its working mechanisms.
dCTP (including other dNTPs, such as dATP, dGTP, dCTP, and dGTP) has several uses inside the body and molecular biology labs. Essentially, they help determine the working mechanisms of organisms’ metabolic functions. Furthermore, they are also used to study the evolution of organisms or the categorization of any new species to a specific family (through DNA sequencing).
Some additional uses and functions of dCTP are explained below:
Cytosine modified at carbon five is a significant epigenetic modification, having an array of roles. Some of its functions in organisms are:
The commercially available dCTP, used in most lab workflows, can be stored at -20 degrees celsius. It’s also highly recommended to refer to the material and safety data sheet (MSDS or SDS) to learn about the quality of the dCTP nucleotide that you are purchasing for your experiment.
Moreover, make sure that the datasheet highlights these four qualities:
dCTP contains three phosphates joined together by phosphoanhydride bonds. These high-energy anhydride bonds release energy when undergoing hydrolysis (a reaction in which water is utilized to break one or more chemical bonds between the molecules).
During DNA replication, the energy released due to the breakage of high-energy bonds is utilized by the enzyme, DNA polymerase, to add dCTP (and other dNTPs) into the newly synthesized DNA strand.
(DNA)n + dCTP ↔ (DNA)n-C + PPi
During the DNA synthesis process:
dCTP is one among four dNTP (deoxynucleotide triphosphates) that form the DNA structure (along with ribose sugar and triphosphate).
dCTP is composed of a pyrimidine cytosine base, a pentose sugar (deoxyribose), and three phosphate groups.
Its preferred IUPAC name—a name given to compounds by IUPAC based on their structure and chemical composition is:
[[(2R,3S,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxy oxolan-2-yl]methoxy-hydroxy phosphoryl] phosphono hydrogen phosphate
Figure: A 2-dimensional structural representation of deoxycytidine triphosphate (dCTP).
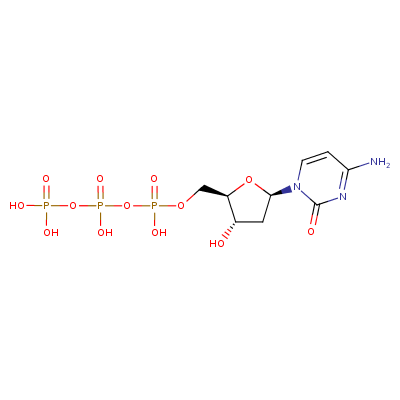
CTP (cytidine triphosphate) should not be confused with dCTP (deoxycytidine triphosphate). Cytidine triphosphate contains ribose with a hydroxyl group at its 2’ position, whereas deoxycytidine triphosphate contains deoxyribose sugars (which has only a hydrogen atom at its 2’position, instead of the hydroxyl group). CTP is used in the formation of RNA, while dCTP is used in DNA.
Deoxycytidine triphosphate (dCTP) is a nucleoside base composed of cytosine, deoxyribose sugars, and three phosphate groups. It’s involved in forming DNA structures, along with other nitrogenous bases that include adenine, guanine, and thymine.
The molecule also acts as a metabolite in several metabolic pathways and is involved in certain diseases. It’s also utilized in labs in a spectrum of in vitro molecular biology workflows, including PCR, DNA sequencing, and determination of nucleotide concentration.
Combining high-quality, lab-grade reagents with cutting-edge equipment enhances the quality, reproducibility, and reliability of researchers’ data and results. At Excedr, we help researchers to outfit their labs with just that.
If you’re working on a budget, our lease program can help you get the technology and equipment it deserves. Get your customized leasing solution now.