
Last Updated on
September 29, 2023
By
Excedr
Western blotting is an analytical technique routinely used in Molecular Biology and Proteomics labs to separate and identify specific proteins from a complex mixture of protein samples.
The technique first separates proteins by molecular weight using SDS-PAGE (electrophoretic protein separation) in the gel matrix in the form of bands. This is followed by the transfer of proteins on nitrocellulose or polyvinylidene difluoride (PVDF) membranes and their incubation with specific antibodies.
The target proteins are detected using detection methods, such as chemiluminescence, fluorescence, or colorimetric techniques. The techniques most similar to western blot analysis include ELISA, immunocytochemistry, and dot-blot analysis, which are used for the quantification of proteins in cell lysates.
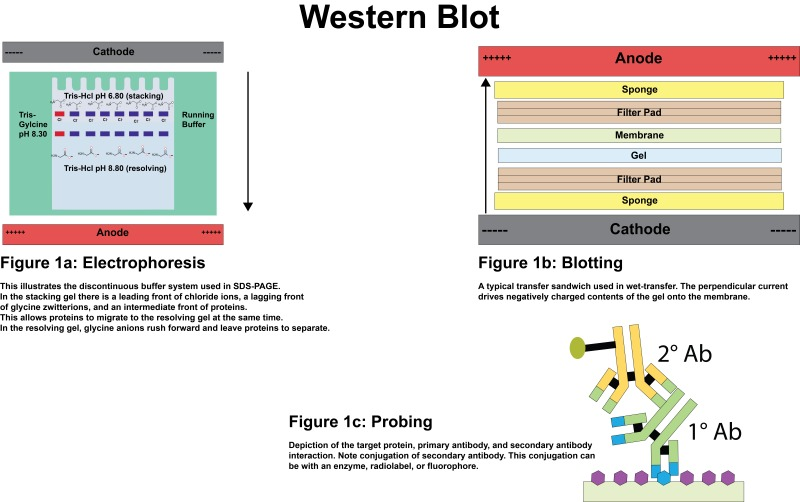
Figure: Western blotting principle.
Western blotting is a frequently employed technique in laboratories and industrial settings. It serves the purpose of identifying and quantifying target proteins and any post-translational modifications they may undergo.
The technique has diverse applications in many areas, such as protein interaction studies, medical diagnostic processes, protein level assessments, drug development, and cell signaling studies.
This article further delves into five types of western blotting transfer systems, how they work, and some troubleshooting tips on the efficient transfer of proteins on membranes.
Excedr can help your lab get the technology and equipment it deserves. With the right equipment, you can accelerate your R&D and achieve milestones faster! Get your customized leasing solution now.
The western blotting procedure was first developed in 1979 by Towbin, et al. They utilized the antigen-antibody interaction for the quantitative analysis of proteins.
Before performing the blotting process, denatured proteins are separated based on their molecular weight using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) technique. The separated proteins in a gel matrix are visible in the form of bands, which are then transferred to immunoblots, such as PVDF or nitrocellulose membranes, in an electric field using the western blot assay.
The efficient transfer of proteins on the membrane requires activation by soaking them in methanol or 95% ethanol. Further, the use of a blocking buffer (usually consisting of non-fat dry milk or 3%-5% BSA (bovine serum albumin)) prevents non-specific binding on immunoblots and ensures quality results. To remove any stuck residue, the membranes are washed using a wash buffer (such as TBST or PBS, consisting of Tris, Tween 20, and NaCl).
The process is followed by incubating the membrane-containing proteins with antibodies specific to the protein of interest. Increasing the exposure time of membranes to antibodies helps in obtaining clearer bands.
In the assay, the primary antibody is designed to target specific proteins. The secondary antibody, which is specific to the primary antibody, is conjugated with specific enzymes or molecules (such as horseradish peroxidase (HRP) and alkaline phosphatase (AP)) based on the chosen detection method. Both the primary antibody and secondary antibody used in the process can either be monoclonal antibodies or polyclonal antibodies.

Figure: An overview of the steps involved in western blotting assay.
The antibody-incubated blotting membrane is then incubated with a specific reaction substrate for the visualization of bands and their quantification for downstream applications. Each band on the membrane is quantified based on the intensity obtained in the imaging process. It gives an idea of the amount of protein in a given sample to study protein expression.
A negative control, a positive control, and a loading control are also used in the process to validate the assay's findings. A negative control is a sample without target proteins, a positive control is a sample with target proteins, and the loading control is a sample of housekeeping proteins, such as beta-actin and alpha-tubulin.
Many different formats of western blotting procedures have been developed based on their purpose in labs or industries. Some commonly used transfer systems used in western blotting assays include:
In a semi-dry transfer system, a sandwich of the gel and membrane is prepared by keeping them between buffer-wetted filter papers. They are placed horizontally on the flat-plate electrodes.
The transfer buffer should properly wet the filter paper-gel-membrane-filter paper sandwich for efficient transfer of proteins. The electrotransfer is commonly performed either at a voltage (10 to 25 V) or constant current (0.1 up to ~0.4 A) for around 10 to 60 minutes.
Generally, it takes around an hour for transfer completion. However, it can be shortened to five minutes by following rapid semi-dry transfer protocols. The major limitation associated with the process is that the large protein may not transfer efficiently on the membrane out of the gel. However, this won't be the case with smaller proteins.

Figure: A semi-dry transfer system.
This transfer method does not involve transfer buffers but uses pre-assembled stacks with proprietary buffer matrices and transfer membranes. Efficient protein transfer is achieved via high ionic density in the gel matrix. Blot distortion is reduced by using copper anodes.
The method allows the complete transfer of proteins in just 10 minutes. However, the only limitation associated with the process is the inability to customize the setup and optimize the protocols based on your requirements or purposes.
In this system, the gel is equilibrated in a transfer buffer and placed between filter papers. This stack is further cushioned with a sponge on both sides and pressed together in the form of a sandwich using a support grid. Then, the stack is placed vertically in a transfer tank filled with a transfer buffer.
The system is well equipped for the efficient transfer of proteins of size 14–116 kDa. However, the transfer works better for smaller size proteins than the larger size. Increasing the transfer time may enhance the quality of the transfer. Note that the smaller-size proteins may be stripped due to over-transfer, especially when the membrane pore size is 0.45 µm.

Figure: Sandwich assembly during wet transfer.
In this system, the proteins in the gel matrix are transferred to nitrocellulose or PVDF membranes through capillary action. The passage of the buffer also takes the proteins from the gel to the membrane. The presence of SDS helps in the efficient transfer of proteins to immunoblots and their subsequent analysis.
Though it’s a time-consuming process, it’s useful for the labs that occasionally perform blotting experiments and aren’t willing to buy a full set of western blot equipment and setup for the same.

Figure: An illustration of the capillary blotting technique.
Western blotting is a high-throughput protein analysis technique used in molecular biology labs for specific protein detection and determination of protein concentration from a mixture.
The technique involves first separating proteins based on their molecular weight using SDS-PAGE. Following that, the protein bands are transferred onto the membrane using a western blotting procedure, which is then incubated with protein-specific antibodies and detected as required.
To achieve reliable results using the protein immunoblotting technique, it is necessary to use high-quality equipment and reagents. Further, sample preparation is critical for efficient protein extraction and protein analysis.
Obtaining advanced equipment for various laboratory procedures can be challenging, especially for newly established labs or those operating with limited financial resources. This is where Excedr steps in with its unique equipment leasing program, catering to labs of all sizes.
Our program empowers researchers and lab founders to access the advanced equipment they require for their studies. The equipment options available through Excedr encompass a wide range, spanning from life sciences and biotechnology tools to clinical and analytical instruments, as well as lab safety equipment.
What sets our program apart is the absence of a hefty upfront payment requirement. Additionally, we take care of the repair and maintenance of the leased equipment, alleviating that responsibility from your shoulders.
Excedr's leasing solution not only simplifies equipment procurement but also enables you to conserve your finances and time. This, in turn, allows you to channel your resources into pioneering research endeavors, accelerating your journey toward breakthroughs and the realization of your goals.
Don’t have the budget to purchase lab equipment outright? Consider leasing through Excedr to save your lab time and money. Browse your leasing options today!